About the SCWO technology
SCWO is short for Supercritical Water Oxidation – an innovative wastewater treatment technology refined by Aquarden. Aquarden’s proprietary SCWO-system SuperOx® makes use of the properties of water in its supercritical phase to dissolve and oxidize even the toughest organic compounds including health-threatening toxins.
How Aquarden’s SCWO-system works
The Aquarden reactor heats and pressurizes wastewater and air to above 374 °C and 221 bar. At these conditions water turns supercritical. Because inorganic salts have very low solubility in this supercritical phase of water, they precipitate and are concentrated as brine. The brine can then be safely disposed of or diluted.
The salt-free supercritical wastewater is then further heated to 450-550 °C, where all organics – including the toughest of harmful toxic compounds – are completely destroyed. The end product is water virtually free of salts and organics. This product is so clean and safe that it can be disposed of through the sewage system, sent to the sea, or reused as process water.
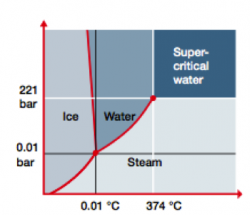
Water has several appearances: Ice, liquid water, or steam. Water can also exist in a fourth state, called supercritical. ‘Supercritical’ is a physical state that water reaches when it is heated above 374 °C and pressurized above 221 bar. Supercritical water readily dissolves organics and oxygen, making it the perfect medium for oxidizing even the most persistent organic pollutants.
Why is SCWO better?
We have developed and refined our SCWO-technology through years of research and development from early concept through to today’s SCWO industrial systems. Our SCWO SuperOx® is a leap forward in cleantech technology because it completely destroys organic compounds while competitive systems only dilute.
SCWO not only cleans better than competitive systems. Studies also document that SCWO is the more cost-efficient alternative to traditional methods, such as incineration, chemical oxidations, and others.
- All organics are eliminated producing harmless CO2 and water
- Compounds containing nitrogen are fully oxidized producing harmless N2
- Compounds containing sulphur oxidize to form sulfate
- Compounds with phosphor convert to phosphate
- Halogens are converted to their corresponding mineral acids
- The process produces no toxic by-products
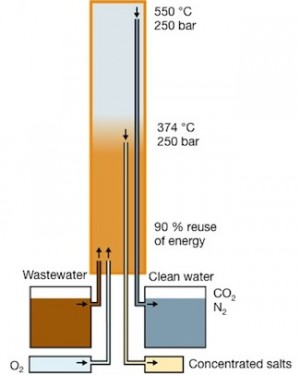
Aquarden’s SCWO reactor SuperOx®: Salts and heavy metals are concentrated and removed at a temperature of around 374 °C. Organics oxidize further up in the reactor at 450-550 °C.
What types of wastewater can SCWO treat?
Aquarden’s SCWO technology is a powerful method for dealing with tough, hazardous organic compounds produced across a variety of industries like Pharmaceuticals, Chemical, Biotech and PFAS removal from airfields etc.
Because SCWO completely destroys all organics, our technology can be applied to a wide variety of wastewater types:
- Medicinal drugs
- Hormones and hormone disrupting compounds
- Pesticides
- Phenols
- Chlorinated phenols
- Solvents
- PFAS substances like PFOS and PFOA
- Etc.


